UK Clinical Study Finds Sky Labs' Smart Ring 'CART-I' More Sensitive Than Apple Watch for AFib Detection
- Published in the international journal Heart Rhythm O2, the clinical study demonstrates that CART-I achieved 84.6% sensitivity – higher than Apple Watch's 69.1%
- Sky Labs also recognized with Korea's top tech honor: IR52 Jang Young-sil Award – Prime Minister's Award
SEOUL, South Korea, April 21, 2025 /PRNewswire/ -- Sky Labs, a health-tech startup based in South Korea, announced that its ring-shaped smart medical device, CART-I (Cardio Tracker), demonstrated higher sensitivity than the Apple Watch in detecting atrial fibrillation (AFib) in a recent clinical study. The study's findings were published in Heart Rhythm O2, a peer-reviewed international journal.
Graphical Abstract, as published in Heart Rhythm O2
"This study offers strong external validation of CART-I's performance in detecting atrial fibrillation," said Jack Lee, CEO of Sky Labs. "Together with CART BP, which focuses on hypertension management, these results demonstrate CART-I's potential to support broader cardiovascular monitoring in the future. We will continue to develop medical devices that deliver both accuracy and convenience for patients."
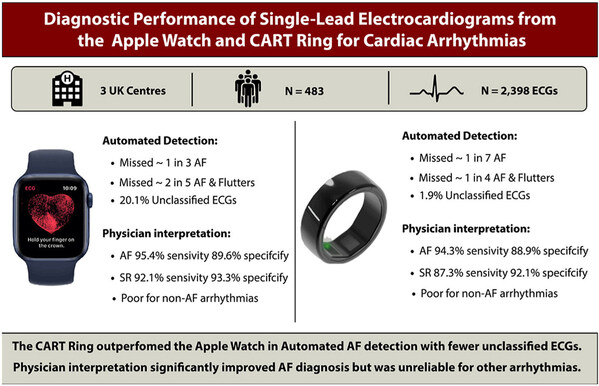
The comparative study, conducted across three hospitals in the UK (Oxford University Hospital, University Hospital Southampton, and Queen Elizabeth Hospital), involved approximately 500 patients and evaluated CART-I and Apple Watch under the same clinical protocol.
Researchers assessed the sensitivity and specificity of each device's automatic AFib detection algorithms using single-lead electrocardiograms (SL-ECG) data, which was subsequently interpreted by physicians. Results showed that Sky Labs' CART-I achieved 84.6% sensitivity, while Apple Watch recorded 69.1% under the same protocol. Both devices also demonstrated high performance in physician interpretation of SL-ECGs: the Apple Watch scored 95.4% sensitivity, and CART-I recorded 94.3%.
Unlike prior studies that focused solely on atrial fibrillation, this research is especially meaningful for its inclusion of a broad range of arrhythmias such as atrial flutter and atrial tachycardia.
Researchers noted, however, that wearable devices alone are not yet sufficient for diagnosing specific arrhythmias such as atrial flutter or atrial tachycardia. Physician interpretation remains essential, and automated algorithms should be considered as supportive tools rather than standalone diagnostic solutions.
Separately, Sky Labs' cuffless ring-type blood pressure monitor, CART BP, was recently awarded the 2024 Prime Minister's Prize at the IR52 Jang Young-sil Awards, one of Korea's most prestigious recognitions for technological innovation. CART BP was selected for fulfilling all four key evaluation criteria: economic value, technological importance, originality, and technological independence.
CART BP is an innovative medical device that transforms traditional blood pressure monitoring by replacing cuff-based, intermittent arm measurements with comfortable, finger-based monitoring over a 24-hour period.
The device received regulatory approval from the Korean Ministry of Food and Drug Safety in 2023 and was approved for national health insurance reimbursement in 2024. As of April 2025, CART BP pro is in use at more than 1,300 hospitals and clinics across South Korea, supporting daily blood pressure monitoring and management.
About Sky Labs
Founded in September 2015, Sky Labs is a leading healthcare startup that has developed CART (Cardio Tracker), a ring-shaped medical device designed for disease monitoring using heart signals collected through optical sensors. Following this, the company developed CART BP, a cuffless, ring-shaped device that enables 24-hour blood pressure monitoring, providing valuable treatment information and making a groundbreaking contribution to improving the quality of life for hypertension patients. Sky Labs has signed an exclusive domestic distribution agreement for CART BP pro with South Korea's Daewoong Pharmaceutical and is preparing for nationwide sales to hospitals, clinics, and general consumers.
Media Inquiries
Edelman Korea SkyLabs@edelman.com
Source: Sky Labs